Ground state and electronic spectrum of Cu(ii) and Cu(iii) complexes of N,N′-1,2-phenylenebis-2-mercaptoacetamide
Abstract
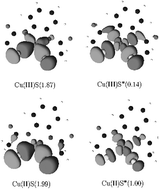
The electronic structure and the UV-vis spectrum of reduced and oxidized model systems of the N,N′-1,2-phenylenebis(2-mercapto-2-methylpropionamide) copper complex have been studied using a multiconfigurational quantum chemical method (CASSCF/CASPT2). The bonds between Cu and the two sulfur

 Please wait while we load your content...
Please wait while we load your content...