A theoretical investigation of the vibrational states of HCO2− and its isotopomers†
Abstract
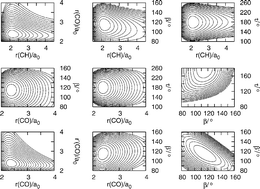
Making use of the coupled cluster variant CCSD(T) and the aug-cc-pVQZ basis set a six-dimensional (6D) potential energy surface has been calculated for HCO2−, a fundamental organic anion. Therefrom, a variety of vibrational term energies and wavefunctions has been obtained by means of the discrete variable representation in an approach termed DVR(6). Calculated wavenumbers of the fundamentals of HCO2− and DCO2− agree with recent experimental values from neon matrix isolation

 Please wait while we load your content...
Please wait while we load your content...