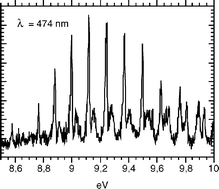
NH2 and NH transitions were identified in dispersed fluorescence spectra obtained by VUV excitation of NH3 at nine different energies between 8.266 and 15.498 eV. Fluorescence excitation (FEX) spectra between 6 and 15 eV were measured at seven specific emission wavelengths for these species. This extends the usual 11.7 eV upper limit of NH3 FEX spectra. Among the results of our work are the following: (i) a clarification of the assignment of the emission carriers; (ii) analysis of the FEX spectra of NH2(Ã
2A1
→ ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2B1) emission and assignment of the features to members of the npe′
← 1a″2, nde″
← 1a″2, nsa′1
← 1a″2 and nda′1
← 1a″2 Rydberg series transitions of NH3; (iii) new information on photodissociation processes leading to formation of NH2(Ã
2A1) and of NH in various excited states, in particular concerning the formation of NH (A 3Π) at 8.266 eV by dissociation of the ã
3A″2 state of NH3, the existence of a potential barrier of 360 ± 70 meV in the dissociation to form NH (c 1Π) at 9.85 eV and its formation from a non-planar state above 11.3 eV; (iv) information pertaining to the heat of formation of the NH radical, which we determine as ΔHf,0°(NH)
= 3.70 ± 0.01 eV, and to the thermochemical limits of the dissociation reactions of NH3.
2B1) emission and assignment of the features to members of the npe′
← 1a″2, nde″
← 1a″2, nsa′1
← 1a″2 and nda′1
← 1a″2 Rydberg series transitions of NH3; (iii) new information on photodissociation processes leading to formation of NH2(Ã
2A1) and of NH in various excited states, in particular concerning the formation of NH (A 3Π) at 8.266 eV by dissociation of the ã
3A″2 state of NH3, the existence of a potential barrier of 360 ± 70 meV in the dissociation to form NH (c 1Π) at 9.85 eV and its formation from a non-planar state above 11.3 eV; (iv) information pertaining to the heat of formation of the NH radical, which we determine as ΔHf,0°(NH)
= 3.70 ± 0.01 eV, and to the thermochemical limits of the dissociation reactions of NH3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2B1) emission and assignment of the features to members of the npe′
← 1a″2, nde″
← 1a″2, nsa′1
← 1a″2 and nda′1
← 1a″2 Rydberg series transitions of
2B1) emission and assignment of the features to members of the npe′
← 1a″2, nde″
← 1a″2, nsa′1
← 1a″2 and nda′1
← 1a″2 Rydberg series transitions of 

 Please wait while we load your content...
Please wait while we load your content...