Consistent supramolecular assembly arising from a mixture of components – self-sorting and solid solutions of chiral oxygenated trianglimines†
Abstract
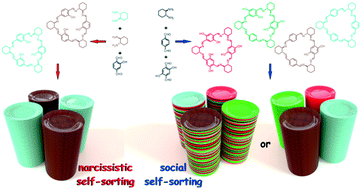
The [3 + 3] cyclocondensation of either oxygen or methoxy group-containing terephthalaldehydes with optically pure vicinal diamines provides triangular macrocycles, whose constitution and symmetry depend on the structure of the aldehyde substrate. Formation of hexa-oxygenated trianglimine is highly stereoselective: that is, the use of racemic diamine yields a racemic mixture of homochiral triangular macrocycles, each having the same absolute configuration at all stereogenic centers. However, no self-sorting is observed when a mixture of non-, mono- and di-oxygenated dialdehydes is subjected to the cyclocondensation reaction with the optically pure diamine. In crystals, the variously oxygenated trianglimines consistently assemble into chiral columnar aggregates and form two- or three-component solid solutions. Some of these assemblies exhibit intracolumnar and/or intercolumnar inclusion properties. While the tris-oxygenated trianglimine crystallizes as a solid solution of molecular isomers, a mixture of uniformly chiral macrocycles that are built of variously oxygenated aldehyde sub-units provides a ternary solid solution deprived of any solvent molecules. For racemic trianglimine, formation of supramolecular columnar assemblies is accomplished by enantioselective self-recognition.


 Please wait while we load your content...
Please wait while we load your content...