“Doubly enantiophobic” behavior during crystallization of racemic 1,5-dihydro-2H-pyrrol-2-one thioether†
Abstract
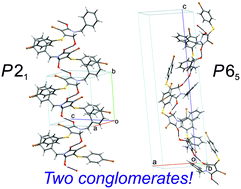
The titled compound 7 demonstrates unusual “doubly enantiophobic” behavior during crystallization. Namely, the mentioned substance forms two polymorphic modifications 7a and 7b that appear to be racemic conglomerates in the space groups P21 and P65 (or P61), respectively. Both 7a and 7b are thermodynamically stable at certain temperature ranges. Hence, γ-lactam 7 is prone to spontaneous resolution of the racemate into the enantiomers in two different ways, which is an exceptional phenomenon. Phase behavior and structure of the crystalline modifications of 7 were investigated by means of single crystal and powder X-ray diffraction, differential scanning calorimetry, temperature-resolved vibration spectroscopy, and hot-stage microscopy.


 Please wait while we load your content...
Please wait while we load your content...