Electrolytic approach towards the controllable synthesis of NiO nanocrystalline and self-assembly mechanism of Ni(OH)2 precursor under electric, temperature and magnetic fields†
Abstract
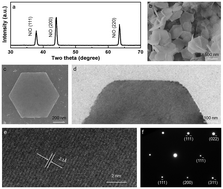
We report that two-dimensional (2D) hexagonal nickel oxide (NiO) nanosheets have been successfully synthesized using the electrolysis of nickel plate as the anode in deionized water. The electrolysis processes first produced 2D nickel hydroxide (Ni(OH)2) nanosheets, which were transformed to 2D NiO by calcination. Contrary to the classical crystallization mechanism, contiguous SEM observations revealed that the Ni(OH)2 were produced via a multistep oriented attachment (MOA) mode, in which the [Ni(OH)6]4− coordination octahedron serves as the building block. Hydrothermal experiments further showed that the technique can be applied to the synthesis of 3D Ni(OH)2 nanoflowers with the aid of introduced Ni(OH)2 nanosheets. A growth mechanism based on the selective-surface adsorption and intercalation of nitrate ions (NO3−) and hydroxyl ions (OH−) as well as the adsorption of free ammonia molecules (NH3), was proposed. The crystal morphologies and thickness can be controlled by a charging magnetic field, suggesting that the crystal growth mechanisms are dominated by micromechanics such as the magnetic dipole force, Van der Waals force and magnetic force.


 Please wait while we load your content...
Please wait while we load your content...