Three-way competition in a topochemical reaction: permutative azide–alkyne cycloaddition reactions leading to a vast library of products in the crystal†
Abstract
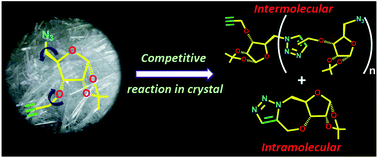
5-Azido-3-O-propargyl-1,2-O-isopropylidene-α-D-ribofuranose (1) showed the possibility of undergoing topochemical azide–alkyne cycloaddition (TAAC) reaction to form 1,5-triazolyl linked polymers in the crystal. Surprisingly, when thermally activated, the molecules reach a conformational equilibrium wherein the native conformer reacts intermolecularly to form a 1,5-triazolyl linkage but the other conformer reacts either intermolecularly to form a 1,4-triazolyl linkage or intramolecularly to form 2. Thus, the TAAC reaction adopts three competing pathways, of which two generate two different types of linkages for chain-growth and the third one generates a chain breaker. While the random occurrence of the chain-breaker reaction ensures the formation of oligomers of different sizes, the permutative occurrence of two types of linkages in any n-mer produces 2n different oligomers of the same size.


 Please wait while we load your content...
Please wait while we load your content...