Low dimensional solids based on Mo6 cluster cyanides and Mn2+, Mn3+ or Cd2+ metal ions: crystal chemistry, magnetic and optical properties†
Abstract
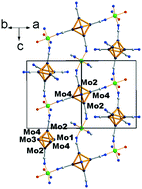
Five new cluster compounds based on [Mo6Bri8(CN)a6]2− and [Mo6Bri6Qi2(CN)a6]n− (Q = S, Se, n = 3, 4) cluster units have been synthesized and characterized. Structures were determined by X-ray single crystal diffraction techniques and measurements of relevant magnetic susceptibility and optical properties were carried out. [trans-Mn(H2O)2][Mo6Br8(CN)6] (1) crystallizes in the orthorhombic system (Imma space group) and contains 2D square-net-layers built-up from [Mo6Bri8(CN)a6]2− and [trans-M(H2O)2]2+ moieties. Csx[trans-(MnIIxMnIII1−x)(H2O)2][Mo6Br6Q2(CN)6] (Q = S (2) and Se (3)) crystallize in the Imma space group as well; their structures are strongly related to that of 1 with a 2D square net of cluster units and transition metals. They are based on [Mo6Bri6Qi2(CN)a6]3− (Q = S, Se) cluster units whose charge is counter balanced by Cs+ as well as Mn2+ and Mn3+ in high spin states. It is evidenced that the x content of the Cs+ counter-cation is equal to that of Mn2+ in order to maintain – along with (1 − x) Mn3+ – 23 valence electrons per cluster and a 3− charge for the cluster unit. The two oxidation states Mn2+ and Mn3+ were confirmed by electron energy loss spectroscopy (EELS) measurements. (H3O)H[cis-Cd(H2O)2][Mo6Br6Q2(CN)6]–H2O (Q = S (4) and Se (5)) crystallize in the trigonal system (P3121 space group) and are based on [Mo6Bri6Qi2(CN)a6]4− (Q = S, Se) cluster units. In contrast to compounds 1–3, owing to the cis-position of the two water molecules around the transition metal, 4 and 5 exhibit a close-packed 3D structure based on an interpenetrated framework of cluster-based chains. In particular, it contains infinite chains alternating [Mo6Bri6Qi2(CN)a6]4− and H+ protons as linkers. Magnetic and optical properties are also reported as well as theoretical calculations to support the structural analysis and physical properties. Structural analogies with [Re6Qi8(CN)a6]4− based compounds are discussed.


 Please wait while we load your content...
Please wait while we load your content...