Structural and computational understanding of weak interactions in “bridge-flipped” isomeric tetrafluoro-bis-benzylideneanilines†
Abstract
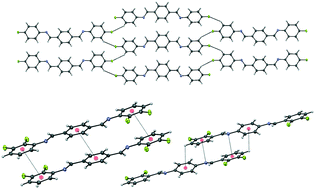
A structural investigation of the crystal structures of nine pairs of tetra-fluorinated “bridge-flipped” bis-benzylideneanilines has been conducted. The crystal packing patterns were analyzed in terms of intermolecular interactions involving the fluorine and nitrogen atoms (i.e., C–H⋯F, F⋯F, C–H⋯π, F⋯π, π⋯π, N⋯π, and C–H⋯N interactions). The stabilization energies offered by these different interactions have been estimated by computational methods using Gaussian 09. Interestingly, strong π⋯π interaction is found only in six exceptional cases, contradicting the general opinion that the π⋯π-stacking synthon is a robust supramolecular synthon. Thus, other intermolecular interactions such as C–F⋯π, C–H⋯π and N⋯π interactions, which are equally strong in stabilizing crystal packing, are also considered to understand the final supramolecular arrangements. These results demonstrate that by changing the orientation of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, one can obtain different supramolecular assemblies through various types of intermolecular interactions. The lattice energy was calculated and decomposed into various components using the semi-classical density sums (SCDS) PIXEL method. A detailed analysis of these interactions based on both experimental and computational methods is provided in the manuscript.
N–, one can obtain different supramolecular assemblies through various types of intermolecular interactions. The lattice energy was calculated and decomposed into various components using the semi-classical density sums (SCDS) PIXEL method. A detailed analysis of these interactions based on both experimental and computational methods is provided in the manuscript.


 Please wait while we load your content...
Please wait while we load your content...