Two novel AIEE-active imidazole/ α-cyanostilbene derivatives: photophysical properties, reversible fluorescence switching, and detection of explosives†
Abstract
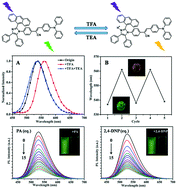
Two novel imidazole derivatives (M-1 and M-2) containing an α-cyanostilbene unit were synthesized and characterized by FT-IR and NMR spectroscopy, mass spectrometry and X-ray crystallography. Compounds M-1 and M-2 exhibited aggregation-induced emission enhancement (AIEE) properties due to intermolecular interactions and the restriction of intramolecular rotation in the aggregated state. The relationships between their structures and photophysical properties were studied via spectroscopic methods and density functional theory (DFT) calculations. It is worth noting that these materials can change their fluorescence emission between green and yellow under gaseous acidic (TFA) and basic conditions (Et3N), and they show reversible acid/base stimulated fluorescence switching behavior in the solid state. The mechanism was further confirmed by 1H NMR spectroscopy in chloroform. In addition, M-1 and M-2 were utilized for picric acid (PA) and 2,4-dinitrophenol (2,4-DNP) detection in a THF–water mixture (fw = 90%) and the detection limits were as low as 10−6 mol L−1.


 Please wait while we load your content...
Please wait while we load your content...