Insights into the polymorphic transformation mechanism of aluminum hydroxide during carbonation of potassium aluminate solution†
Abstract
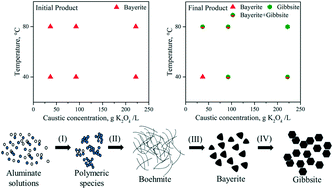
In this study, the carbonation process, in which potassium aluminate solution reacted with CO2/KHCO3 solution under different caustic concentrations (37.0 g K2Ok L−1, 92.5 g K2Ok L−1 and 222.0 g K2Ok L−1) and temperatures (40 °C and 80 °C), was investigated. The precipitation products were characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD) and Mastersizer. Bayerite was found to be present in the initial crystalline products, regardless of the temperature, caustic concentration and carbonation method and also, it was present as the predominant phase under most experimental conditions, which is fairly different from previous studies that reported the dependence on temperature or concentration. The transformation from bayerite to gibbsite was apparently promoted with an increase in temperature and caustic concentration. The polymorphic transformation during carbonation of potassium aluminate solution is discussed kinetically and structurally according to the calculated nucleation rate and is supposed to be: Al(III)-containing cluster → boehmite → bayerite → gibbsite. The growth rate of bayerite was always several orders of magnitude higher than that of gibbsite, which led to the formation of the final products in a temperature- and caustic concentration-dependent manner. At a relatively high temperature and caustic concentration, the reaction was thermodynamically controlled and the most thermodynamically stable phase, namely gibbsite, was the predominant final product. However, at a low temperature and caustic concentration, the reaction was kinetically controlled and bayerite was the predominant product. It is concluded that the change in thermodynamic stability is not likely for bayerite and gibbsite in the present study. However, precise research on the possibility of the stability crossover between Al(OH)3 polymorphs is worthy.


 Please wait while we load your content...
Please wait while we load your content...