Polymorphs of daidzein and intermolecular interaction effect on solution crystallization†
Abstract
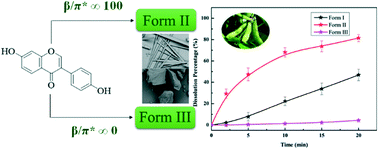
Daidzein (DAID), known as a phytoestrogen, is an important bioactive flavonoid isolated from soybean and pueraria. However, its poor solubility and low dissolution rate limit its clinical application. With the aim of finding a form with fit-for-purpose physicochemical properties, a comprehensive solid-state screening experiment was performed. As a result, three polymorphs were discovered and the single crystal structure of stable form II was revealed for the first time. Form II was obtained in solvents with high β and π* values, while form III was produced under opposite conditions. To clarify this phenomenon, intermolecular interactions were investigated through single crystal structure analysis, solvent property analysis and computational methods. The results demonstrated that the compact arrangement of DAID and the solvents used played a critical role in solution crystallization. Moreover, the physicochemical properties of these three polymorphs were fully characterized, and the powder dissolution behavior was determined in pure water and pH 1.2 and pH 6.8 buffer solutions. The newly discovered form II exhibited better thermal stability, lower moisture sorption and faster dissolution rate compared with the other forms.


 Please wait while we load your content...
Please wait while we load your content...