Influence of mole-ratio on the coordination behaviour of a ditopic N2O2S2-macrocycle: endo/exocyclic silver(i) coordination polymer exhibiting desolvation-induced SCSC transformation†
Abstract
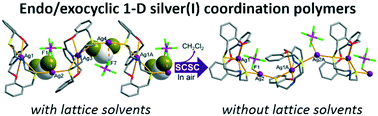
Mole-ratio dependent formations of the macrocyclic silver(I) complexes with different coordination modes and topologies are reported. When one equivalent of AgPF6 was reacted with an N2O2S2-macrocycle (L), a typical endocyclic monomer complex [Ag(L)]PF6 (1) was isolated. Moreover, the use of three equivalents or above amount of AgPF6 afforded a one-dimensional (1-D) coordination polymer {[Ag4(L)2](PF6)4·2CH2Cl2}n (2a), in which the endocyclic monomer complex units are linked by different silver(I) atoms outside the macrocyclic cavity to form a unique endo/exocyclic infinite chain via the –endoAg–S–exoAg–S– bond linkages. Upon removal of the dichloromethane molecules in the crystal lattice of 2a in ambient conditions, conversion of the coordination geometry of the exocyclic Ag atom from linear (2a) to trigonal plane (2b, desolvated product) as well as sliding of the 1-D chains were observed in a single-crystal-to-single-crystal manner.


 Please wait while we load your content...
Please wait while we load your content...