Understanding the role of water in 1,10-phenanthroline monohydrate†
Abstract
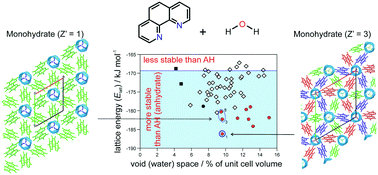
The solid forms emerging from an experimental screening programme of 1,10-phenanthroline (o-phen), a heavily used bidentate ligand, and interconversion pathways of its two neat forms, the monohdyrate (Hy1) and four solvates with acetone, chloroform, dichloromethane and 1,2-dichloroethane are described. The solvates, identified and characterised with thermoanalyical methods, are unstable when removed from the mother liquor and desolvate at room temperature depending on the relative humidity (RH) to anhydrate I° (AH I°) or transform to Hy1. At ambient conditions Hy1, a stoichiometric channel hydrate, is the thermodynamically most stable o-phen solid form. The enthalpically stabilised Hy1 melts at 102 °C or dehydrates to AH I° at RH < 10% at 25 °C. The potential energy difference between Hy1 and AH I° was calculated to be approx. 15 kJ mol−1. The second anhydrate polymorph (AH II) can be obtained from the quench cooled melt of o-phen, but is unstable at ambient conditions and transforms within minutes to either AH I° or Hy1. The two neat polymorphs are enantiotropically related and water-free o-phen transforms to Hy1 at RH > 16%. The structural and stability features of the solid forms, in particular Hy1, are unravelled by a combination of experimental (thermal analysis, moisture sorption/desorption and storage experiments, infrared spectroscopy and powder X-ray diffraction) and computational modelling (crystal structure prediction and lattice energy calculations), providing a consistent picture why o-phen forms a very stable Z′ = 3 channel hydrate.


 Please wait while we load your content...
Please wait while we load your content...