The halogen bonding proclivity of the ortho-methoxy–hydroxy group in cocrystals of o-vanillin imines and diiodotetrafluoro-benzenes†
Abstract
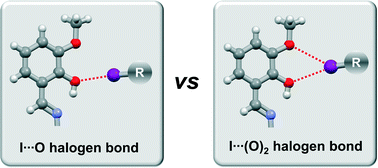
In this work, we describe novel halogen bonded o-vanillin imine cocrystals. As cocrystal coformers, we selected perfluorinated diiodobenzenes, 1,2-, 1,3- and 1,4-diiodotetrafluorobenzene, as halogen bond donors. To explore the halogen bonding proclivity of the o-vanillin ortho-methoxy–hydroxy group, two imines were produced by condensation of o-vanillin with amines containing hydrophobic residues that have no additional potential halogen bond acceptor sites other than the o-vanillin oxygen atoms. We have prepared four cocrystals by both one-pot three-component solvent-free synthesis and the conventional solution-based method. The results of our work show that the ortho-methoxy–hydroxy group of the o-vanillin moiety is a good halogen bond acceptor and is capable of forming predictable halogen-bonded synthons. In the studied cocrystal structures, a wide range of varieties of this synthon were observed, including bonds with a single acceptor (either the methoxy O atom – in two structures, or the hydroxy O atom – in one), ‘slightly bifurcated’ bonds with a dominant (hydroxy) and ancillary (methoxy) acceptor, and a geometrically unusual asymmetrical bifurcated bond.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...