Controllable assembly of Cu(ii) coordination compounds based on a flexible zwitterionic benzimidazole–dicarboxylate ligand: synthesis, structural diversity, reversible SCSC transformation and magnetic properties†
Abstract
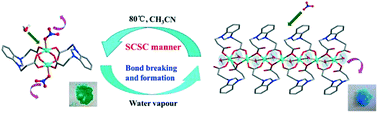
A systematic investigation on the assembly of a flexible zwitterionic ligand, 1,3-bis(2-carboxyethyl)benzimidazolium (HL), with various Cu(II) salts under different conditions afforded nine coordination compounds: [Cu2L2(NO3)2]·CH3CN (1), [Cu2L2(H2O)2](NO3)2·5H2O (2), {[CuL(H2O)](NO3)}n (3), [Cu4L4(OH)2(H2O)2](NO3)2·10H2O (4), [Cu2L2(H2O)2](ClO4)2 (5), [CuL(H2O)3]2SO4·7H2O (6), [Cu(HL)2Cl2] (7), [Cu2L2Cl2]·2H2O (8) and {[Cu2L2Cl2]·2H2O}n (9). Compounds 1 and 8 are neutral paddle-wheel shaped dinuclear clusters with either nitrate or chloride as the axial ligand, while 2 and 5 are composed of identical [Cu2L2(H2O)2]2+ cationic units displaying a paddle-wheel motif with an axially coordinated aqua ligand but different counter anions. Compound 3 exhibits a 1D linear structure, where two adjacent Cu(II) centers are triply bridged via two carboxylate groups plus one aqua ligand. Compound 4 is a tetranuclear cluster supported by two μ3-bridged OH− groups and four carboxylate bridges. Compounds 6 and 9 are mononuclear species constructed from the dicarboxylate ligand in two different coordination modes. Compound 9 assumes a 1D looped chain topology with paddle-wheel cluster nodes propagated by pairs of flexible linkers. A close examination revealed that the conformational freedom of the flexible dicarboxylate ligand as well as the labile binding modes of its two side arm carboxylates is sensitive to the reaction conditions, thus resulted in the different structural types of these coordination compounds. The solvent-induced reversible single crystal-to-single crystal (SCSC) transformation between 1 and 3 could be easily achieved via the shuttle movement of NO3− outward and inward of the Cu(II) coordination sphere accompanied by a visible color change. Upon dissolution–recrystallization, the irreversible or reversible structural transformations among 2, 3, 4 and 6 could be driven by subtly controlling some factors such as solvent, pH, temperature and counter anion. The magnetic measurements for compounds 2–4 demonstrated that their magnetic behaviors are governed by antiferromagnetic interactions despite the fact that dissimilar exchange coupling pathways between neighboring Cu(II) ions are available in these three compounds.


 Please wait while we load your content...
Please wait while we load your content...