Supramolecular chirality and symmetry breaking of fluoride complexes of achiral foldamers†
Abstract
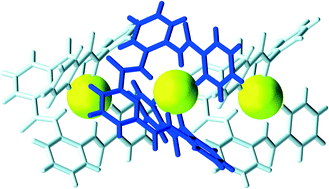
Aromatic oligoamide foldamers containing a central pyridine-2,6-dicarbonyl motif are partially preorganized to favor the binding of fluoride anions. In the solid state, the foldamer-fluoride complexes form achiral, polar and chiral crystal structures depending on the chemical structure of the foldamer. One of the six foldamers studied here, a C2v symmetrical foldamer (1), formed repeatedly chiral crystal structures when crystallized with tetra-butylammonium fluoride, showing supramolecular bulk chirality and symmetry breaking in crystallization.


 Please wait while we load your content...
Please wait while we load your content...