A series of six-membered lanthanide rings based on 2,2-bis(hydroxymethyl)-2,2′,2″-nitrilotriethanol: synthesis, crystal structures and magnetic properties†
Abstract
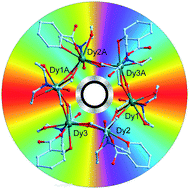
By utilizing a solvothermal method, a series of six-membered cyclic lanthanide(III) clusters, {Gd6(H3L)6(benzoate)6}·2H2O·C7H8 (1), {Tb6(H3L)6(benzoate)6}·2H2O·C7H8 (2) and {Dy6(H3L)6(benzoate)6}·2H2O (3), have been constructed with the pentol ligand, 2,2-bis(hydroxymethyl)-2,2′,2″-nitrilotriethanol (H5L). Similar hexa-nuclear ring structures were observed in each of the crystal structures. In addition, magnetization studies indicated that Gd6 displays large magnetic entropy changes in low temperature regions. What is particularly interesting about this ring is the presence of slow magnetic relaxation behavior under a zero dc field, indicating a non-magnetic ground state with a net toroidal magnetic moment. Compared to the other reported Dy6 clusters, modulation of the ligand fields, the presence of amine coordinating sites and the subsequent electron density of the Dy centers along with the utilized pentol ligand might be indeed responsible for the improved barrier to magnetic relaxation.


 Please wait while we load your content...
Please wait while we load your content...