Hydrogen- and halogen-bond cooperativity in determining the crystal packing of dihalogen charge-transfer adducts: a study case from heterocyclic pentatomic chalcogenone donors†
Abstract
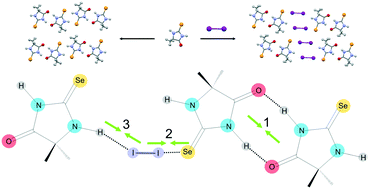
Three new molecular CT adducts with dihalogens, based on 5,5-dimethyl-2-thiohydantoin (dth) and 1,5, 5-trimethyl-2-thiohydantoin (mdth) donor molecules have been synthesised and characterised by single crystal X-ray diffraction and their crystal structures compared with those of previously published analogous compounds to assess the role of molecular shape of the donors, and cooperativity and competition between hydrogen-bonds (HBs) and halogen-bonds (XBs) in defining the observed supramolecular architectures at the solid state. The study of the role played by these factors was supported by several computational tools. The structural feature at the base of the crystal packings observed is the formation of dimers of donor molecules via complementary N–H⋯O or N–H⋯S HBs. These dimers are arranged in space in 2D architectures via further interactions involving the S/Se⋯I–I/Br XBs. Interestingly, in most of the cases the XB interactions strengthen upon formation of the self-assembled H-bonded dimers, indicating a favourable cooperativity effect which is at the base of the supramolecular architectures formed.


 Please wait while we load your content...
Please wait while we load your content...