Self-assembly of conducting cocrystals via iodine⋯π(Cp) interactions†
Abstract
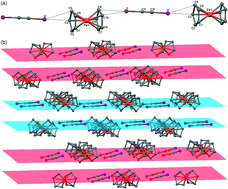
Conducting crystals of alternating ferrocene and diiodoacetylene units assembled into supramolecular 1-D chains via I⋯π(Cp) halogen bonds were prepared and structurally characterized. Their structure and conductivity were compared with the ferrocene cocrystals with phenyliodoacetylene (C6H5C2I) and 1,4-di(ethynyliodo)phenylene. Electrical conductivity measurements of the ferrocene-based cocrystals revealed comparatively high electron–hole type conductivity in the ferrocene (FcH)–diiodoacetylene (C2I2) pair, a 3-order decrease in combination with the 1,4-di(ethynyliodo)phenylene linker, and it is virtually extinguished with the monotopic 1,2-phenyliodoacetylene. The increased electric conductivity of compound 1 is rationalized in terms of a weak charge transfer through the I⋯πCp halogen bonds in the [Cp–Fe–Cp⋯I–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–I⋯]n polymeric chains.
C–I⋯]n polymeric chains.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...