Five 1D to 3D Zn(ii)/Mn(ii)-CPs based on dicarboxyphenyl-terpyridine ligand: stepwise adsorptivity and magnetic properties†
Abstract
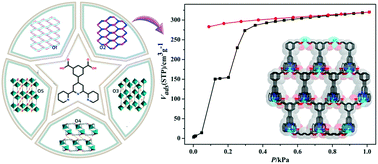
Five coordination polymers, {[Zn(dtp)]·H2O} (1), {[Zn(dtp)]·2CH3OH·NMP} (2), {[Mn2(dtp)2(H2O)4]·12(H2O)} (3), {[Mn(Hdtp)(Cl)]} (4) and {[Mn(dtp)(H2O)]·1.5H2O} (5) with different dimensional structures, have been solvothermally synthesized by utilizing the H2dtp ligand (H2dtp = 4′-(3,-5-dicarboxyphenyl)-2,2′:6′,2′′-terpyridine). All complexes are characterized by single-crystal X-ray diffraction, elemental analysis, IR spectra, thermogravimetric analysis (TGA) and powder X-ray diffraction (PXRD). The Zn(II)-based complexes 1 and 2 display a 2D wavy layer structure and a 3D porous framework with a 1D channel, respectively. The three Mn(II)-based complexes 3–5 exhibit two 1D chain and one 2D layer structures, respectively. Complexes 1 and 2 reveal their luminescence properties, whereas complexes 3–5 display an antiferromagnetic exchange. Meanwhile, an unusual stepwise adsorption for CO2 was also observed in 2.


 Please wait while we load your content...
Please wait while we load your content...