Calcite passivation by gypsum: the role of the cooperative effect†
Abstract
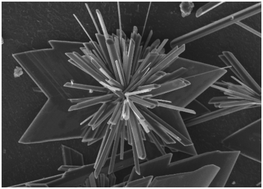
The overgrowth of gypsum (CaSO4·2H2O) on calcite (CaCO3), used as a substrate, has been obtained in order to verify the hypothesis of a crystallographic fit between the two phases. Lattice coincidences show that many orientations of gypsum with respect to calcite can occur. Experimentally, only two among these are fulfilled during the early stages of growth, the selection criterion reasonably being the adhesion energy. Moreover, it has been shown that the presence of calcium carbonate as a specific surface impurity in solution modifies the growth habit of gypsum acting on the growth rate and surface morphology of some gypsum forms. At high concentration, the calcium carbonate in solution promotes the twinning of gypsum according to the Montmartre law.


 Please wait while we load your content...
Please wait while we load your content...