Ionic cocrystals of molecular saccharin†
Abstract
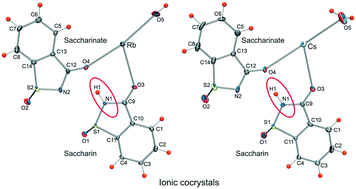
Saccharin is a cyclic sulfimide whose sodium salt, commercially available under the trade name “Sweet'N Low”, is one of the most commonly used artificial low-calorie sweeteners and is also the main sugar substitute in the diet of diabetics. Being an acid (pKa = 1.6), it is readily deprotonated in solution and affords solid ionic salts or coordination compounds with transition metals where its conjugate base (saccharinate ion) displays a wealth of coordination modes. Here we report the first two examples of ionic cocrystals of molecular saccharin where saccharin exists as a neutral species and an ion in the same crystal. With rubidium and cesium cations, saccharin forms isomorphous solid hemihydrate salts. When saccharin is supplied in excess to the reaction mixture, neutral saccharin molecules are stoichiometrically incorporated into both crystals and stable isomorphous ionic cocrystals are obtained. The formation of ionic cocrystals is unprecedented and adds a new aspect to the rich crystal chemistry of this artificial sweetener.


 Please wait while we load your content...
Please wait while we load your content...