Compositional introduction of lithium ions into conductive polyoxovanadate–surfactant hybrid crystals†
Abstract
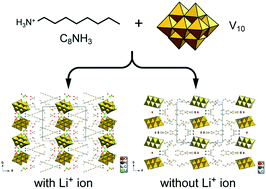
Polyoxovanadate–surfactant hybrid layered crystals were successfully synthesized as single crystals by employing a primary alkylammonium cation, octylammonium ([C8H17NH3]+, C8NH3). Two types of hybrid crystals with the formulae [C8H17NH3]6[V10O28]·2H2O (C8NH3–V10) and [C8H17NH3]4Li2[V10O28]·4C2H5OH·6H2O (C8NH3–Li–V10) were obtained by different synthetic procedures. Changing the synthetic conditions enabled the precise introduction of lithium cations into the polyoxovanadate–surfactant hybrid crystals according to compositional control. C8NH3–V10 contained a discrete [V10O28]6− anion, while C8NH3–Li–V10 was composed of a [V10O28]6− anion associated with two lithium cations formulated as {[Li(H2O)3]2[V10O28]}4−. The conductivities of C8NH3–V10 and C8NH3–Li–V10 were investigated under anhydrous conditions at intermediate temperatures.


 Please wait while we load your content...
Please wait while we load your content...