Ligand-controlled formation of covalently modified antimoniomolybdates and their photochromic properties†
Abstract
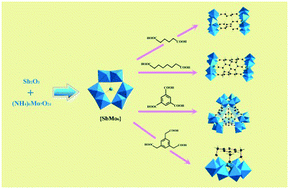
Four inorganic–organic hybrids based on multicarboxylic acids and antimoniomolybdates, namely, (NH4)4(C3N2H5)4H4[(SbMo6O21)2{OOC(CH2)4COO}3]·7H2O (1), (NH4)8(C3N2H5)2H2[(SbMo6O21)2{OOC(CH2)6COO}3]·8H2O (2), (NH4)24[(SbMo6O21)4{C6H3(COO)3}4]·8H2O (3), and (NH4)6[(SbMo6O21){C9H9(COO)3}]·3H2O (4) have been synthesized under conventional solution conditions. Studies reveal that these compounds are monomers, dimers or tetramers, depending on the structures of the coordinated carboxylic acids. Also, the photochromic properties of these compounds were studied and compared, and based on the different carboxylic acids they displayed great disparities in the coloration speed under irradiation; the speed sequence is: 4 {benzene-1,3,5-triacetic acid (BTA)} > 2 (suberic acid) > 1 (adipic acid). It is indicated that compared to the applied aliphatic acids, the aromatic carboxylic acid ligands will facilitate the enhancement of the coloration speed of organic-POM hybrids.


 Please wait while we load your content...
Please wait while we load your content...