Occurrence of 3D isostructurality in fluorinated phenyl benzamidines†
Abstract
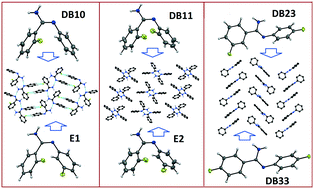
In the current study, we report the existence of 3D isostructurality behaviour in pairs of isomeric molecules existing as E–Z isomers. The polymorphic forms E1 and E2 of (E)-2-fluoro-N′-(3-phenyl) benzimidamide are individually isostructural with (E)-2-fluoro-N′-phenylbenzimidamide and (E)-2-fluoro-N′-(2-fluorophenyl) benzimidamide, respectively. Furthermore, a similar compound, (Z)-3-fluoro-N′-(4-fluorophenyl) benzimidamide, is isostructural with (Z)-4-fluoro-N′-(4-fluorophenyl) benzimidamide. The structural feature has been analyzed in terms of the nature and energetics of the equivalent supramolecular building blocks associated with the presence of various intermolecular interactions in the crystal packing. The crystal packing similarities have been investigated quantitatively via XPac analysis and the energy vector model. It is noteworthy to observe the presence of a rarely observed N–H⋯F interaction which plays an important role in the crystal packing of [(Z)-3-fluoro-N′-(4-fluorophenyl) benzimidamide]/[(Z)-4-fluoro-N′-(4-fluorophenyl) benzimidamide]. The NCI isosurface signifies the “attractive” nature of the N–H⋯F and N–H⋯π interactions and the different types of C–H⋯F dimeric motifs. An analysis of the two-dimensional fingerprint plots also provides a quantitative understanding of the occurrence of isostructurality in these compounds.

 Please wait while we load your content...
Please wait while we load your content...