Ring size effect on the solid state assembly of propargyl substituted hexa- and octacyclic peptoids†
Abstract
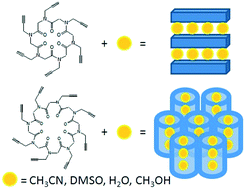
The investigation of the solid state assembly of propargyl substituted hexa- and octacyclic peptoids highlights the effect of ring size in determining the packing arrangement of the macrocycles. A layered arrangement is obtained in the case of the hexacyclic peptoid 1 and a tubular arrangement in the case of the octacyclic peptoid 2. Guest molecules either intercalate between the layers as in 1 or are located within the peptoid nanotube as in 2.


 Please wait while we load your content...
Please wait while we load your content...