Insights into the opening of DNA-like double-stranded helices in lamivudine duplex IV and the first polymorph of this drug†
Abstract
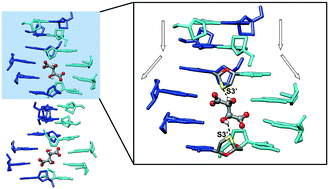
Here we report an enantiospecific assembly of the drug lamivudine with D-tartaric acid mimicking the opening of a DNA duplex. In this structure, named lamivudine duplex IV, the D-tartrate counterions are in charge of the opening of the two base-paired helically-stacked strands, in analogy to the role of helicase in nature. This multicomponent crystal between lamivudine and D-tartaric acid therefore provides insights into the molecular basis of nucleic acid strand separation. The hydroxyl group from either D-tartrate or a serine residue of helicase is a hydrogen bond donor to the sugar ring from a nucleoside, which suggests a base unpairing role of such an interaction. Furthermore, the enantiospecificity of such a phenomenon can be stated as being the result of the duplex opening through only the D enantiomer. When L-tartaric acid was used to attempt the opening of the lamivudine duplex, only a monohydrate salt without any base-pairing motif was obtained. The first anhydrous polymorph of lamivudine was also obtained in the course of our cocrystallization screening between lamivudine and L-tartaric acid. This new polymorph was named lamivudine form IV and is made up of only the drug, as observed in its commercial polymorphic form II. Lamivudine form IV consists of base-paired dimers held together by uncommon N–H⋯N hydrogen bonds, but they are not helically stacked due to the absence of an enantiospecific strand opener.

 Please wait while we load your content...
Please wait while we load your content...