Low- and high-temperature oxidation of Mn1.5Al1.5O4 in relation to decomposition mechanism and microstructure†
Abstract
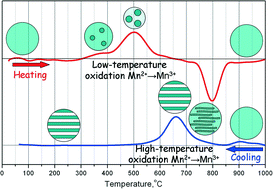
Spinel-like Mn1.5Al1.5O4 is an unstable compound whose decomposition is induced by partial oxidation of Mn2+ ions in the temperature range of 300–800 °C in air. According to XRD, two spinel-like phases, Mn0.4Al2.4O4 and Mn2.8Al0.2O4, are formed during both cooling and heating of Mn1.5Al1.5O4. In this paper, we have studied microstructural changes of Mn1.5Al1.5O4 to understand its decomposition mechanism. During heating, low-temperature oxidation takes place and decomposition proceeds via a nucleation and growth mechanism. As a result slightly Al-doped β-Mn3O4 nanoparticles are formed on the surface of the parent spinel particles. On the contrary, cooling leads to high-temperature oxidation that results preferably in spinodal decomposition and formation of alternating Mn- and Al-rich lamellas of nanosized thickness. The present study provides a fundamental reference for the nanostructure design of a Mn–Al–O system and probably some other ones subjected to oxidative decomposition.

 Please wait while we load your content...
Please wait while we load your content...