Crystal engineering of a hybrid metal–organic host framework and its single-crystal-to-single-crystal guest exchange using second sphere coordination†
Abstract
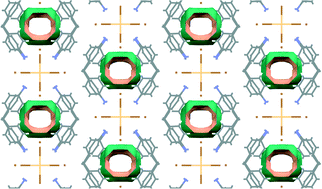
By means of crystal engineering principles, a new second sphere coordination adduct has been synthesized using charge-assisted hydrogen bonds and self-assembling [CdBr6]4−and Br− anions with a V-shaped diprotonated cation. The second sphere adduct is homologous to a copper network, forms a host framework with 1D channels and has good thermal stability (200 °C) upon guest release. The [CdBr6]4− anion shows Jahn–Teller distortion as revealed by X-ray crystallography. Guest exchange via a single-crystal-to-single-crystal manner is reported. The guest behavior is different to that of the homologous Cu adduct due to the pore size effect.

 Please wait while we load your content...
Please wait while we load your content...