Crystal structures of hydrated rare-earth bis(trifluoromethylsulfonyl)imide salts†
Abstract
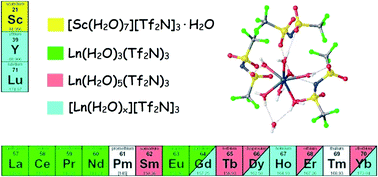
Crystals of hydrated bis(trifluoromethylsulfonyl)imide (Tf2N) salts of the naturally occurring trivalent rare-earth ions were grown from aqueous solution and analysed by X-ray diffraction. Their structures can be classified into three main groups. The lighter lanthanides La–Gd (except Sm) have a cubic crystal structure with the formula Ln(H2O)3(Tf2N)3, which has Tf2N anions coordinating in a bidentate fashion. The middle and heavy lanthanides (Sm, Tb, Dy, Er, Yb) have a monoclinic crystal structure with formula Ln(H2O)5(Tf2N)3, where the Tf2N anions are coordinating in a monodentate fashion. The heavy lanthanides Gd–Lu (except Tb, Tm) crystallise with very large unit cells making structural identification difficult. Partial structure solution of one of these compounds indicates that the structures contain fully hydrated Ln3+ ions and very disorderd Tf2N anions. Y was found to behave as the heavy lanthanides and Sc was found in a crystal structure of formula [Sc(H2O)7][Tf2N]3·H2O. In addition, La was found in a hexagonal crystal structure of formula La(H2O)3(Tf2N)3·2.5H2O, when a small amount of residual HTf2N was present during crystallisation.

 Please wait while we load your content...
Please wait while we load your content...