Tetrazolate–azido–copper(ii) coordination polymers: tuned synthesis, structure, and magnetic properties†
Abstract
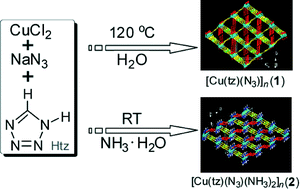
As the first example of using a parent tetrazole and an azide together in preparing magnetic complexes, two novel tetrazolate–azido-bridged Cu(II) coordination polymers, [Cu(tz)(N3)]n (1) and [Cu(tz)(N3)(NH3)2]n (2) (tz = tetrazolate), have been synthesized through the reaction of Htz, CuCl2, and NaN3 under hydrothermal conditions and in ammonia solution at room temperature, respectively. Single crystal X-ray structure analysis reveals that the two complexes possess distinct three-dimensional (3D) framework structures and can be topologically described as a 3-connected srs (SrSi2)-type net and a 4-connected cds (CdSO4)-type net, respectively. Both tetrazolate and azide groups also have different linkage modes in the two complexes. 1 contains end-on (EO) type azide bridges and 3-connected tetrazolate, while 2 possess end-to-end (EE) azide linkers and 2-coordinated tetrazolate. Magnetic measurements indicate that antiferrimagnetic interactions dominate between Cu(II) ions in the two complexes with the corresponding magnetic coupling constant being J = −41.0 cm−1 in 1 and J = −8.62 cm−1 in 2.

 Please wait while we load your content...
Please wait while we load your content...