Acid-induced Zn(ii)-based metal–organic frameworks for encapsulation and sensitization of lanthanide cations†
Abstract
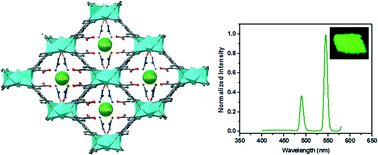
Two Zn(II)-based metal–organic frameworks, namely, [Zn3(Hbptc)2(DMF)2]·2DMF(1) and [Zn5(bptc)3(H2O)]((CH3)2NH2)2(2), (H4bptc = 3,3′,5,5′-biphenyltetracarboxylic acid, DMF = N,N′-dimethylformamide), have been synthesized using different amounts of nitric acid under mixed-solvothermal conditions. 1 and 2 display different coordination geometries and donor ligands for the Zn2+ ions. In 1, only three carboxylic acid groups of H4bptc take part in the construction of the three-dimensional (3-D) framework with Zn2+, and one remains uncoordinated that can bind to other metal ions. Postsynthetic exchange of 1 with Ln3+ cations demonstrates that 1 can effectively capture metal cations and sensitize the visible-emitting Ln3+.

 Please wait while we load your content...
Please wait while we load your content...