Structural chemistry and selective CO2 uptake of a piperazine-derived porous coordination polymer†
Abstract
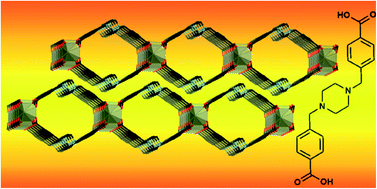
A new piperazine-derived ligand has been prepared and used to synthesise a porous coordination polymer which displays selective carbon dioxide uptake after solvent exchange and thermal activation. The ligand N,N′-bis(4-carboxyphenylmethylene)piperazine H2L1 was prepared from piperazine in three steps and good yield. A structure containing the deprotonated form K2L1·2H2O was determined and consists of a three-dimensional coordination polymer containing inorganic K2(COO)2(OH2) layers separated by the long organic bridging linker. The free compound H2L1 displays a one-dimensional hydrogen-bonded polymeric structure in the solid state with hydrogen bonding interactions between carboxylic acids and piperazine groups tightly linking molecules together. The two-dimensional polymeric complex [Zn3(L1)2(OH)2]·2DMF·0.5H2O 1 was prepared and analysed in the solid state to reveal tubular one-dimensional channels which, when activated by solvent exchange and evacuation, displayed selective affinity for CO2 over N2 and H2.


 Please wait while we load your content...
Please wait while we load your content...