Assembly of dinuclear copper(ii) secondary building units into polymeric complexes: crystal structures and magnetic properties†
Abstract
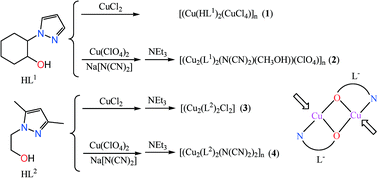
Four Cu(II) complexes, the one-dimensional [Cu(HL1)2(CuCl4)]n (1), {[Cu2(L1)2(N(CN)2)(CH3OH)](ClO4)}n (2), dinuclear [Cu2(L2)2Cl2] (3), and two-dimensional layered [Cu2(L2)2(N(CN)2)2]n (4) were synthesized by Cu(II) salts (CuCl2·2H2O or Cu(ClO4)2·6H2O), alkanol bidentate ligands trans-2-(1-pyrazolyl)cyclohexanol (HL1) or 3,5-dimethyl-N-hydroxyethyl-pyrazolyl (HL2) together with sodium dicyanamide. For complex 1, the bridging chlorine atoms of the tetrahedral [CuCl4]2− moieties are located at the Jahn–Teller axis of the elongated octahedral Cu(II), forming an alternate elongated-octahedral-tetrahedral Cu(II) chain complex. For complexes 2, 3 and 4, the alkyl oxygen atoms of the deprotonated ligands HL1 and HL2 bridge two Cu(II) ions, yielding a dinuclear Cu(II) unit [Cu2(Lx)2]2+ (x = 1, 2), and one- or two-dimensional complexes via bridging dicyanamide ligands. Complex 1 shows weak ferromagnetic coupling, whilst complexes 2–4 display strong antiferromagnetic coupling or are even nearly diamagnetic due to the large Cu–O–Cu bond angles.

 Please wait while we load your content...
Please wait while we load your content...