Constructing various metal–organic frameworks by mixed pyridine–acylamide and carboxylate ligands: ring-like or helical building blocks†
Abstract
A new pyridine–acylamide ligand, L![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) L
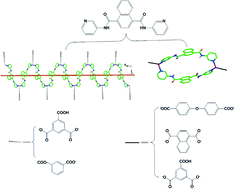
L![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N1,N4-di(pyridin-3-yl)naphthalene-1,4-dicarboxamide, has been synthesized. Based on L, four types of carboxylate ligands as co-ligands and two metal ions (Zn(II) and Cd(II)), seven new metal–organic frameworks have been synthesized and structurally characterized. Compounds 1 and 2 are composed of SBU I (a helical {Zn(L)}n chain), while compounds 3–7 are composed of SBU II (a M2L2 metallocycle). In 1, with Hbtc2− anions connected to the neighboring Zn(II) ions of one SBU I, an infinite 1D chain is formed, while in 2, the neighboring SBUs I are bridged by ip2− anions forming a 2D structure. With SBUs II extended by oba2− ligands, compound 3 shows a 2D network. Compound 4 exhibits a 1D infinite chain with SBUs II bridged by nap2− ligands. Compounds 5 and 6 are isomers in which SBUs II are extended by nap2− ligands to afford a 2D network. In compound 7, SBUs II are linked together by Hbtc2− ligands featuring an infinite 1D chain. All of these compounds have a 3D framework governed by hydrogen bond interactions. Comparing all the compounds, it can be seen that the structural characteristics of the L ligand and organic counteranions simultaneously play an important role in their construction. Moreover, their thermostability and photoluminescence properties were explored.
N1,N4-di(pyridin-3-yl)naphthalene-1,4-dicarboxamide, has been synthesized. Based on L, four types of carboxylate ligands as co-ligands and two metal ions (Zn(II) and Cd(II)), seven new metal–organic frameworks have been synthesized and structurally characterized. Compounds 1 and 2 are composed of SBU I (a helical {Zn(L)}n chain), while compounds 3–7 are composed of SBU II (a M2L2 metallocycle). In 1, with Hbtc2− anions connected to the neighboring Zn(II) ions of one SBU I, an infinite 1D chain is formed, while in 2, the neighboring SBUs I are bridged by ip2− anions forming a 2D structure. With SBUs II extended by oba2− ligands, compound 3 shows a 2D network. Compound 4 exhibits a 1D infinite chain with SBUs II bridged by nap2− ligands. Compounds 5 and 6 are isomers in which SBUs II are extended by nap2− ligands to afford a 2D network. In compound 7, SBUs II are linked together by Hbtc2− ligands featuring an infinite 1D chain. All of these compounds have a 3D framework governed by hydrogen bond interactions. Comparing all the compounds, it can be seen that the structural characteristics of the L ligand and organic counteranions simultaneously play an important role in their construction. Moreover, their thermostability and photoluminescence properties were explored.

 Please wait while we load your content...
Please wait while we load your content...