Facile solvothermal synthesis of BiOCl–TiO2 heterostructures with enhanced photocatalytic activity†
Abstract
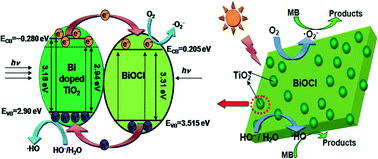
BiOCl–TiO2 heterostructures with different Bi to Ti molar ratios were synthesized via a facile one-pot solvothermal process. The as-synthesized BiOCl–TiO2 heterostructures included TiO2 nanoparticles deposited on the surface of BiOCl nanoplates. X-ray photoelectron spectroscopy results revealed the TiO2 of BiOCl–TiO2 heterostructures doped with Bi3+, reduced the band gap energy of the sample. The photocatalytic efficiencies were evaluated by degradation of methylene blue dye under simulated sunlight illumination. Results indicated that the BiOCl–TiO2 heterostructures with Bi : Ti = 1 : 3 exhibited the highest photocatalytic efficiency, which is 73.5 times and 8.5 times higher than that of single phase BiOCl and TiO2, respectively. In addition, the radical species involved in the degradation process have been investigated by scavenger experiments. Finally, a new mechanism for the photocatalytic action of the BiOCl–TiO2 heterostructures was put forward.

 Please wait while we load your content...
Please wait while we load your content...