Ordered LiMPO4 (M = Fe, Mn) nanorods synthesized from NH4MPO4·H2O microplates by stress involved ion exchange for Li-ion batteries
Abstract
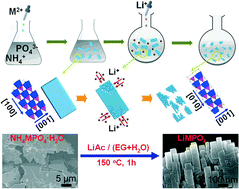
Nanoengineering has proven to be an effective strategy to improve the electrochemical properties of LiMPO4 (M = Fe, Mn). In this work, we report an ‘up-to-down’ way to synthesize LiMPO4 nanorods through an ion-exchange route using pre-prepared NH4MPO4·H2O microplates as the precursor and LiAc as the Li source. The similarity in crystal structure between NH4MPO4·H2O and LiMPO4 allows the occurrence of the ion-exchange reaction, while their structural difference induces lattice stress that breaks the NH4MPO4·H2O microplates to form interconnected, orderly arranged LiMPO4 nanorods. This work provides a general method to synthesize nanosized LiMPO4 from microsized NH4MPO4·H2O by taking advantage of their crystallization characteristics. The nanoscaled LiMPO4/C composites exhibit promising electrochemical properties after carbon coating.

 Please wait while we load your content...
Please wait while we load your content...