Comparative study of LiMnPO4 cathode materials synthesized by solvothermal methods using different manganese salts
Abstract
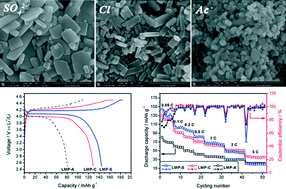
The effects of anions on the particle size, phase impurity, crystal structural, and crystal growth orientation of olivine LiMnPO4 prepared via the solvothermal synthesis method are systematically studied. LiMnPO4 cannot be obtained for the precursor containing NO3− anions due to its strong oxidizing ability in acid environment. The SO42− anion may facilitate the growth of high-index planes of the LiMnPO4 crystals owing to its higher charge number. The Ac− anion with larger volume may have a significant atomic-scale template effect, thus the particle size of LiMnPO4 is greatly limited and the crystal growth is inclined to the equilibrium state. The LiMnPO4 samples, prepared from MnSO4, MnCl2 and Mn(Ac)2, deliver an initial capacity of 145, 129 and 81 mAh g−1, respectively. Among them, the sample synthesized from MnCl2 can maintain 91% of its initial capacity after 200 cycles at 2 C, due to its high purity and crystal orientation growth with ac planes.

 Please wait while we load your content...
Please wait while we load your content...