Synthesis and structural analysis of alkylammonium sulfoisophthalates: cation size and geometry effects on the hydrogen bonded network†
Abstract
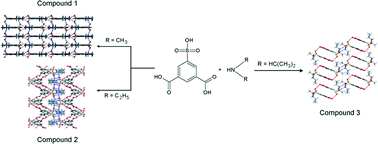
The solid-state organization of alkylammonium sulfoisophthalates has been analysed with respect to competing carboxylic–carboxylic and carboxylic–sulfonate interactions and preferred geometry of the amine–sulfonate interactions. All three compounds studied display layered structures with sulfoisophthalic monoanions arranged into sip-layers and alkylammonium cations crosslinking them. The size and geometry of the cation influences in a considerable way the hydrogen bond organization in the monolayers. The 2D anionic network is flat in compound 1, and becomes strongly corrugated in the presence of diethylammonium ion in compound 2. However, the bulky diisopropylammonium ion interrupts the network dimensionality and the monolayer in 3 is formed from sip chains, which are hydrogen-bonded into ribbons. Multiple weak interribbon interactions, established between the electron clouds of the aromatic rings and the lone pairs of O atoms, as well as, between the π electrons of the carbonyl double bonds hold together the neighbouring ribbons in the monolayer of 3. The common bridging–bridging ammonium–sulfonate synthon is observed in crystals 1 and 3. However, the ammonium–sulfonate synthon in crystal 2 appears to be a unique coupling of bridging–bridging and bifurcated interactions.

 Please wait while we load your content...
Please wait while we load your content...