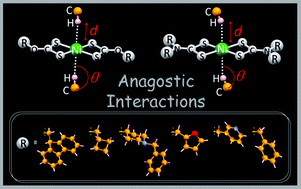
Six new homoleptic complexes of the general form [Ni(L2)] [L = L1 (9-fluorenylmethyl)xanthate (1); L2 (cyclobutylmethyl)xanthate (2); L3 (1-benzyl-4-hydroxypiperidine)xanthate (3); L4 (N-benzyl-N-methylpyridyl)dithiocarbamate (4); L5 (N-methylfuryl-N-methylpyridyl)dithiocarbamate (5); L6 (N-benzyl-N-methylfuryl)dithiocarbamate (6)] have been prepared and characterized by microanalysis, spectroscopy (IR, 1H and 13C NMR, UV-Vis) and their structures have been elucidated by X-ray crystallography. In all complexes the metals are four coordinate with square planar coordination geometries although in the crystal structures of 2 and 4 significant C–H⋯Ni anagostic intermolecular interactions in axial positions are found which involve the metal atom in chain motifs. As far as we are aware 2 and 4 are unique examples of homoleptic metal dithio complexes exhibiting such type of anagostic interactions. In the remaining complexes (1, 3, 5 and 6) supramolecular frameworks are stabilised by S⋯S, C–H⋯S, C–H⋯π and π⋯π non-covalent interactions. All six complexes are weakly conducting (σrt = 10−11–10−8 S cm−1; Ea = 0.37–1.40 eV) and show semiconductor behaviour in the 303–393 K temperature range.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...