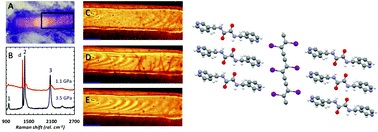
Diiodobutadiyne forms cocrystals with bis(pyridyl)oxalamides, based on halogen bonds between the pyridine groups of the host and the iodoalkynes of the guest. These interactions align the diyne for topochemical polymerization to form poly(diiododiacetylene) or PIDA. To induce polymerization, the crystals are subjected to pressures of 3.5 GPa or above. Previously, we reported spectroscopic evidence of this pressure-induced polymerization, but attempts to recover single crystals after pressure treatment were unsuccessful. Here we present direct structural evidence of clean single-crystal to single-crystal polymerization in these cocrystals. The structure of the polymer cocrystal was solved from single-crystal diffraction data and is supported by high pressure in situ Raman spectroscopy. Careful analysis of the structural changes suggests that increasing pressure changes the packing of host molecules, and that the flexibility of the pyridine ring orientation enables the polymerization. The new sigma bonds of the polymer form at the expense of the halogen bonds in the starting cocrystal; after polymerization, the iodine atoms are no longer ideally located for strong halogen bonding with the host.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...