Thermodynamic and crystal growth kinetic study of uranium peroxide
Abstract
In the processing of uranium ores, the uranium is recovered from mill leach solutions by
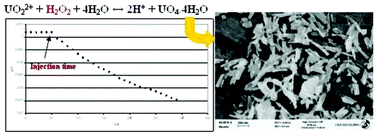
- This article is part of the themed collection: Crystallisation: From Fundamentals to Application

 Please wait while we load your content...
Please wait while we load your content...