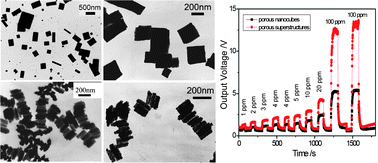
Morphological regulation of nanomaterials is essential for their properties and potential applications, and many strategies have been developed. The great influence of anions on the morphologies of nanomaterials was discovered recently by being adsorbed selectively on a specific crystalline plane of material to tune its growth habit. In this work, the effect of anions on the morphologies of In(OH)3 nanostructures was studied and a series of In(OH)3 nanostructures have been obtained by controlling the species and concentrations of the presenting anions in hydrothermal system. In(OH)3 nanocubes and nanorods were obtained by using indium chloride as indium source, whereas nanoflakes were produced in the indium nitrate case. Moreover, adding appropriate amount of sodium halides into the hydrothermal reaction could convert nanoflakes into nanocubes and nanorods, while adding sodium nitrate into the hydrothermal system of indium nitrate could lead to the formation of superstructures constructed by aligned nanoflakes. By calcining these In(OH)3 nanostructures, porous In2O3 nanostructures with inherited morphologies were obtained. The gas sensors fabricated by these porous In2O3 nanostructures exhibited excellent and morphology-dependent sensitivities for ethanol vapor, implying their great potential in ethanol detection.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...