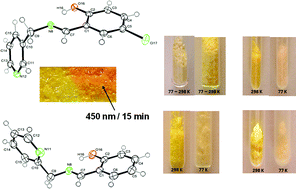
Five N-(5-chloro)salicylidene aminomethylpyridine derivatives were successfully synthesized and their structural and solid state optical properties analyzed. Yellow crystalline powders of CH22L33 and CH2222L3–43–43–43–4Cl present both thermo- and photochromism whereas CH22L22 and CH222L444 do not show any switching properties, as probed by diffuse reflectance spectroscopy. Contrary to structural–optical expectations, the non-photochromic CH22L22 molecule (P21/n) shows an open crystal structure, and photochromic molecules CH2222L3–43–43–43–4Cl (P21/c) present a closed crystal packing, revealed by single crystal X-ray diffraction, which is typical of exclusively thermochromic molecules. After UV irradiation, trans-keto* emission observation in CH22L22 and CH222L444 indicates the unexpected formation of the trans-keto form in these non-photochromic anil molecules. Radiative relaxation of the trans-keto* form is in addition detected, for the first time, by fluorimetry for all N-salicylidene molecules of the series, whatever their switchable chromic properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...