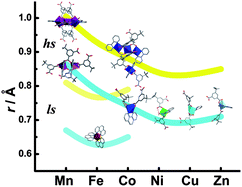
18 coordination compounds of Mn2+, Fe2+, Co2+, Ni2+, Cu2+ and Zn2+, with mixed organic ligands 5-tert-butylisophthalic acid, 2,2′-bipyridine or phenanthroline, have been hydrothermally synthesized and characterized. Structure analysis shows that the ionic radius of a metal ion determines the form of the metal centre and the structure of the product. Large metal ions tend to have higher coordination numbers, connect to more bridging ligands and form multinuclear metal centres. On the contrary, small metal ions tend to connect to fewer bridging ligands, and act as mononuclear metal centres for discrete coordination compounds or low dimensional coordination polymers. The influences of other factors such as molar ratio of reactants, pH and temperature, have also been discussed. The magnetic properties of the temperature dependent cobalt compounds have been investigated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...