Organic molecules induced crystal transformation from a two dimensional coordination polymer to chain-like structures†
Abstract
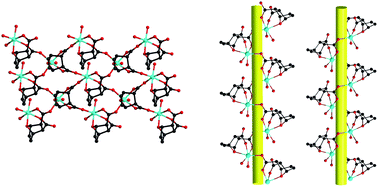
Using exo-7-oxabicyclo-[2.2.1]-5-heptene-2,3-dicarboxylic acid (H22L), three Mn(II) complexes formulated as [Mn(L)(H2O)]·H2O (1), [Mn2(L)2(H2O)4]·(C6H6O)·5H2O (2) and [Mn(L)(H2O)2]·0.5(C6H6O2) (3) have been synthesized and structurally characterized by single-crystal X-ray diffractions. Complex 1 shows a uninodal 3-connected 2D layer with 63-hcb topology. In complexes 2 and 3, similar alternating left-handed and right-handed helical chains are observed. Complex 1 is transformed into complexes 2 and 3 upon the attack of

 Please wait while we load your content...
Please wait while we load your content...