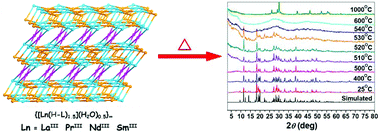
A series of three-dimensional (3-D) lanthanide–organic frameworks (LnOFs), with the general formula [Ln2(H–L)3(H2O)]∞ (H–L = N-protonated 2,6-dihydroxypyridine-4-carboxylate; LnIII = LaIII for 1, PrIII for 2, NdIII for 3, and SmIII for 4), have been synthesized under hydrothermal conditions. X-Ray diffraction and energy dispersive X-ray spectroscopy (EDS) analyses reveal that complexes 1–4 are isostructural and exhibit the unique 3-D coordination framework with (4,5,7)-connected network topology, in which the H–L ligands adopt the μ4- and μ5-bridging fashions. Remarkably, thermogravimetric analysis of the crystalline materials shows their exceptionally high thermal stability (up to 530 °C), which should originate from the dense nature of the packing lattices and the unusual network structures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...