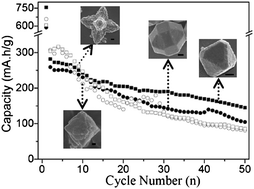
Crystalline materials with a well-defined morphology and/or a narrow size distribution might exhibit a specific shape- and/or size-dependent performances. In the first instance, the catanionic surfactants of anionic sodium dodecyl sulfate (SDS) and cationic cetyltrimethylammonium hydroxide (CTAOH) were added as crystal modifiers into the 60 °C reaction systems of copper chloride and sodium hydroxide for the syntheses of cuprous oxide (Cu2O). Then, the reversible reaction activity of crystalline Cu2O with metal lithium was conducted to investigate its electrochemical performance as rechargeable lithium ion battery anodes. The presence of SDS-rich catanionic surfactants could induce the formation of polyhedral Cu2O structures with 8 triangular {111}, 6 square {100}, and 12 rectangular {110} faces outside, while the presence of CTAOH-rich catanionic surfactants, especially the doping methanol in CTAOH, led to the generation of hexapod-shaped Cu2O mesostructures with tiny nanoparticles on these symmetrical branches. At a discharge–charge cycling current of 80 mA g−1, the 26-faceted Cu2O crystals with rough {110} faces displayed an initial capacity of 756 mA h g−1 and a reversible capacity of 280 mA h g−1 at the first cycling. In comparison with the electrochemical performance of Cu2O hexapod-shaped mesocrystals at the same cycling current, the 26-faceted crystals of Cu2O could be capable of remaining a relatively high capacity (∼145 mA h g−1) and keeping an excellent Coulombic efficiency (∼100%) over 50 discharge–charge cycles. As a whole, the catanionic surfactants at different anionic/cationic molar ratios were used as additives to highlight the secondary nucleation and growth mechanism for the formation of Cu2O, then, the resulting 26-faceted crystallites and hexapod-shaped mesoparticles were separately used as active materials in the assembled Cu2O/Li half-cells to study their shape-dependent electrochemical performances.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...