Insight into the crystal lattice formation of brookite in aqueous ammonia media: the electrolyte effect
Abstract
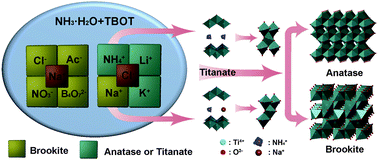
A feasible approach to synthesize pure brookite was achieved in an aqueous ammonia system via a hydrothermal process. By hydrothermal treatment of the titanate that directly hydrolyzed from

 Please wait while we load your content...
Please wait while we load your content...